Stress and dose interactions;
Diet as modulator or mode of exposure; Developmental status/age and toxicity; C
4, 10, 30, 34
Course Comments
While I will post a Discussion
topic this week, you should spend some time reading and evaluating all the various
penultimate project files that have been posted. Enter a reply with your comments and
suggestions for each of the projects; this should be done over the next week so
people can respond to and incorporate those comments in their final project
submissions.
Communications will continue to be
made to the Prometheus Administrators so that materials become easier to load
and read in the site.
If anyone has not responded to my
query concerning the final exam format, please do so at your earliest convenience.
Stress and Dose Interactions
Stress Axes
What is stress
physiologically?
* Internal stimuli (loss of
homeostasis, chemical signal imbalance)
* External stimuli (environmental ‑‑
heat, light, pressure, sound, biological interactions between organisms,
interpersonal/behavioral)
Note either source of stimulus can
be acute or chronic. Stress gives rise to supra‑ or ab‑normal
systemic activities. At the cell level these may involve heat shock protein
expression, changes in membrane potentials and ion balance, changes in cellular
metabolism, changes in gene expression, changes in receptor numbers, etc. At
the tissue level, these translate into changes in chemical or hormonal
sensitivity, "irritability," capacity to respond or function. At the
system level the changes give rise to alterations in excitability and chemical
signal output. Examples include:
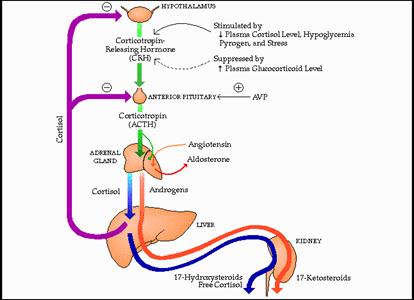
1. Adrenal axis/adrenal cortex:
CRH, cortisol, DHEAS levels rise thereby increasing blood glucose levels,
enhancing lipolysis, suppressing immune functions among other end results.
2. Adrenal medulla: norepinephrine
& epinephrine rise thereby altering blood pressure and cardiac rate.
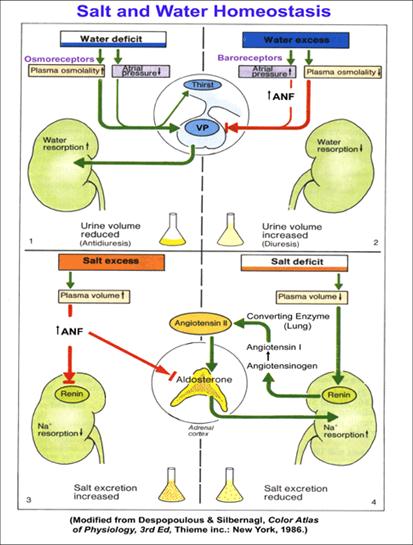
3. Kidney: responds to vasopressin
by increasing water retention; responds to a fall in renal perfusion by
increasing renin output resulting in rises in angiotensin I & II and
aldosterone (from the adrenal cortex, glomerulosa
layer) with subsequent increases in sodium retention and blood pressure.
Note that stress enhances both VP
output and constrictions of blood flow to the kidney juxtaglomerular apparatus
leading to over production of renin, angiotensins,
and aldosterone. Pharmaceutical
preparations targeted at stress-related or sedentary life-style-related high
blood pressure often inhibit angiotensin converting enzyme (ACE inhibitors) so
as to suppress the salt and water retention caused by over activity of this
axis. Diarrhetics
counter the salt retention actions of the axis while beta blockers target
vascular smooth muscule and increase renal
perfusion. Agents that augment salt
and/or water retention accentuate any blood pressure elevations and may
stimulate chronic high blood pressure and the cardiovascular accidents that
accompany that condition.
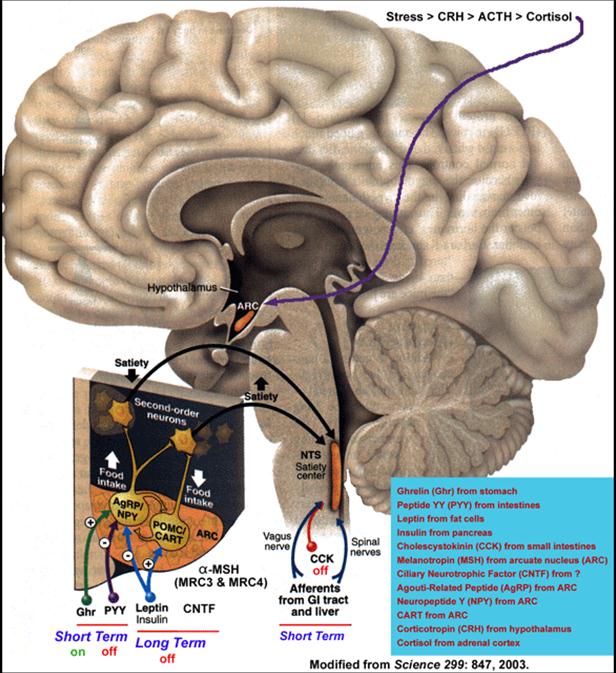
4. CNS: may increase output of
opiate peptides to suppress nociceptive stimuli; may decrease NPY and other
peptides while increasing CART, CRH, and other peptides in the leptin pathways resulting in suppression of apetite; may increase output of vasopressin, dopamine,
serotonin or other neurotransmitters causing alterations in both sympathetic
and parasympathetic nerve transduction pathways.
5. Thyroid: may increase output of
thyroxine causing generalized increases in metabolic
rate and increased thermal output; under chronic stress the reverse may occur;
thermal control is compromised under both situations.
6. Immune system: interleukins,
CRH, bradykinin, and prostaglandins may all be released
causing alterations in adrenal, thyroid, CNS and other functions. Note that the
kinins and prostaglandins may stimulate smooth muscle
contractions of the vascular endothelium or even organ walls ‑‑
stomach, intestine, uterus (Clearly infection is an environmental stressor.)
Note several normal biological
processes utilize stress as a signal. Disease has been mentioned. Should toxins
alter any of the steps involved in these signaling pathways and intracellular
signal cascades, they have the potential to either block or exacerbate the
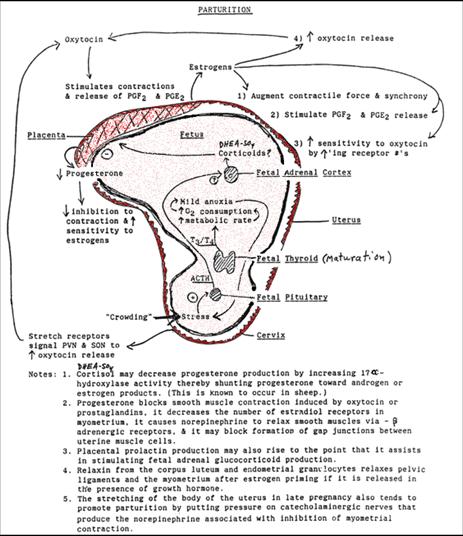
stressful condition. The birth process involves stress on both the offspring
and the mother. In the offspring near term the maturing thyroid axis and
general rapid growth of the fetus brings on a generalized anoxic stress.
Generalized "crowding" by the maternal organs including the pelvic
bones stimulate the fetal adrenal axis to produce CRH, ACTH, and resultant
increased cortisol (and DHEAS). Placental CRH is also rising at this time and
helps reinforce this stimulus of the fetal adrenal axis. The adrenal steroids
alter the metabolism of steroids and prostaglandins in the placenta.
Progesterone production falls in favor of estrogen production while the
placental inactivation of prostaglandins that normally occurs during pregnancy
is inactivated. The increased estrogens, and decreased progesterone levels
favor increases in oxytocin receptor numbers in the maternal myometrial cells. They also increase the maternal systemic
stimulation of the posterior pituitary production of oxytocin and the myometrial cells' formation of gap junctions. The oxytocin
actions both directly and mediated by formation of prostaglandins in myometrial cells stimulate coordinated contractions of the
maternal myometrium and ultimately lead to completion of parturition.
Other systems also contribute to
the process: pudendal nerve compression during late
pregnancy decreases output of norepinephrine which normally decreases myometrial contractility; opiates are increased
dramatically during birth to act as endogenous pain suppressors.
Additional mechanisms may also
contribute in this case. For example, progesterone levels which peak at or near
term for most mammals may act via glucocorticoid receptors during the latter
part of pregnancy to alter glucose metabolism, immune responses, and possibly
even help decrease expression of prostaglandin inactivating enzymes, though
this latter mechanism has yet to be shown.
Shock is another, if extreme,
example of systemic stress that may be accelerated or induced by toxicant
actions. Generally breakdown of kidney function or of osmotic tissue barriers
leads to decrease in blood pressure, decline in cardiac rate and output, a
decrease in oxygen and glucose perfusion of the brain and other organs, CNS depression,
respiratory depression, and systemic failure. Any toxicant accelerating this
chain of events may cause shock if given in high enough dose.
Reactive Oxygen
Currently, the major path for actions
of toxicants in causing tissue effects not related to uptake by particular
receptors or via indirect impacts on cellular metabolism or cell division,
involves the concept of oxidative stress. This is covered in some depth in
Hodgson and Smart, Introduction to Biochemical Toxicology, 3rd Ed, 2001,
John Wiley & Sons, Inc.: New York, NY, 235‑253.
Specific elements of the concept
are covered in the links provided below as well as in C&D Chapter 3. The
concept entails the idea that cells are damaged as a result of the breakdown in
balance of reduction and oxidation within the cells. If oxidative conditions
arise as a result of the unchecked intracellular production of peroxide or
superoxide radicals, or reactive nitrogen species, these molecules can covalently
alter other molecules, including macromolecules, within the cell. Oxidized
lipids or proteins will compromise membrane functions so mitochondrial or cell
membrane potentials may be altered. Intracellular signaling may also stimulate
production of "cell death" signals causing the initiation of the
events leading to apoptotic cell death.
The production and redox cycling
of glutathione plays a key role in maintaining intracellular oxidative balance.
Adequate levels of other reductive species such as retinoids,
ascorbic acid, tocopherol, and reduced nicotine
adenine dinucleotides help maintain the balance.
Overabundance of metals like iron or copper can be problematic. While any toxicant that requires rapid P450 mediated metabolism
increases the endogenous risks for overproduction of superoxide and peroxide
species that may act as proximal reactive intermediates in causing cellular
oxidation or macromolecular modification. Unless the reductase
activities and other repair processes can keep up, the cell, minimally, and/or
the tissue involved, may undergo damage from oxidative stress.
Oxidative stress and radiation:
http://www.sysbio.org/sysbio/stress/
Apoptosis Induction
Ultimately many of these stressors
tend to lead to cell death via apoptosis by means of activating mitochondrial
damage and triggering of the cytochrome c dependent cell death pathways, or via
cell surface receptor-mediated pathways (e.g., FAS/FASL, TRAIL). Tissue damage may thus be silent with respect
to the reticuloendothelial system. However, if sufficiently widespread it can
still lead to a diminution of tissue function.
Loss of cardiac capacity, especially on reperfusion with well-oxygenated
blood, would be an example of this kind of damage. Note that developmentally and during tissue
growth and repair, growth factors like EGF or NGF often trigger production of
proteins like Bcl which block the induction of
apoptosis via the Bax mediated pathways. This makes sense when the environment of
affected cells is being exposed to high concentrations of oxygen via perfusion
by blood passing through new vessels and capillaries. Or when active tissues are
generating oxidative products such as lipid metabolites that would otherwise
cause cellular compromise.

So the end-products of stress are
not only temporary compromises in tissue function mediated by temporary changes
in tissue production of hormones or by alterations in the capacity to respond
to hormones. They may also include
actual structural and functional changes caused by the local intoxication
effects of endogenously produced reactive oxygen species. Although these may be
temporary and acute changes. They
may also become chronic and permanent via induction of apoptotic cell death in
sufficiently large numbers of cells in sensitive tissues.

Diet as a Modulator or Mode of
Exposure
Water & Food as Vehicles
of Exposure
While we often think of food and
water as simply nutriative, they also may contain components
or contaminants that may have toxic properties.
Since no animals can survive without water and nutrients, we have no
choice but to consume these materials.
Our only recourse to limit their potential for contributing to overall
toxic insults is to eliminate or limit those components or contaminants we know
to be associated with toxic properties.
This stands in contrast to the approaches needed to limit toxic
exposures in the workplace and living environment where many toxic sources can
be totally avoided without consequence, and to the use of pharmaceuticals which
undergo extensive testing prior to manufacture and sale. The limitation of food and water associated
intoxication is a focus for public health with respect to humans and a focus
for environmental and ecotoxicology with respect to other organisms including
plants and animals. These foci have led
to a series of laws governing food production and sales (Federal Food, Drug,
and Cosmetic Act and amendments: http://www.uvm.edu/nusc/nusc237/ffdcatc.html
(or see) http://www.fda.gov/opacom/laws/lawtoc.htm;
Federal Insecticide, Fungicide, and Rodenticide Act and amendments: http://www.epa.gov/region5/defs/html/fifra.htm) and
protection of water sources (Federal Water Pollution Control Act and
amendments: http://laws.fws.gov/lawsdigest/fwatrpo.html, http://www.usdoj.gov/crt/cor/byagency/epa1251.htm
; Safe Drinking Water Act and amendments: http://www.epa.gov/safewater/sdwa/sdwa.html
, http://www4.law.cornell.edu/uscode/42/300f.html
) as well as to the establishment of government agencies (Food and Drug
Administration, Environmental Protection Agency) that implement these
laws. Much of this material is covered
in C&D chapters 30 & 34.
For regulatory purposes food is
considered a mixture including both constituents and additives. Constituents are legally unregulated. Additives are of three types: unintentional
additives which are unregulated if incidental; unintentional additives which
are regulated if they occur as a byproduct of normal handling of the foodstuff
(e.g., plastic monomers from containers); or, they are intentional additives
that were added to modify the quality, form, or handling properties of the food
to which they were added, in which instance they will be either regulated or
termed “generally recognized as safe” (GRAS).
Note that none of these
classifications are initially based on toxic qualities. They are a practical breakdown of food
components. Constituents may be every
bit as toxic as any intentional additive.
For example, peanut proteins or oils may be highly allergenic yet they
are intrinsic elements of any foods derived from peanuts. Likewise, the goitrogens
found in some yam species are chemicals produced by the yams themselves and the
toxins inherent to certain cycads or fishes are avoided only by careful
preparation of the foodstuffs. Vitamin
A poisoning can occur with over-consumption of liver, especially when taken raw
from predatory species like polar bear.
Yet all these instances are nonregulated.
By contrast leaching of metals
like tin or lead or plastic monomers such as phthalate esters from food
containers or food handling equipment is regulated and monitored by the
regulating bodies.
These food contaminants include:
pesticides, packaging constituents, metals, animal drugs, food toxins (of
plant, animal, or mycotic origin), and bacterial
contaminants (including pathogens that are virulently infectious & those
that are highly enterotoxic). Screening for some of these materials is the
basis for many of the activities of the field staff of the Food and Drug
Administration, e.g., the meat inspectors in meat processing plants and the
fish inspectors on the docks and in the fish markets in New England.
Intentionally added constituents
are subdivided essentially by historic accident. Those already in foodstuffs at the time of
adoption of the controlling legislation were segregated into two classes: those that were regulated and those that
were GRAS. Materials became GRAS if they
had been used prior to the legislation and had been demonstrated to be safe by
testing, or, more likely for most traditional additives, by lack of negative
experience during prior use in the public food supply, i.e., safe according to
human epidemiological evidence from prior use experience. Thus, many additives were “grandfathered” in
as GRAS on relatively slim evidence.
Common food additives include at
least 30 categories of compounds including:
Preservatives (e.g., MSG, BHT,
BHA)
Nutriative Supplements (e.g., Vitamins A, C, D, E, K; iodine)
Antioxidants (e.g., Sulfites,
EDTA, PABA, vitamins A, C, E)
Nonnutriative Supplements (e.g., oils, sweeteners)
Fillers
Flavors
Colors
Scents
Processing agents (e.g.,
cornstarch or flour to make sweetened cereals less sticky)
Many of the chemicals or mixtures
that fall in these categories are covered by the GRAS designation. But there is now recognition that this may
not always be an appropriate designation.
Note that colors, flavors, and
scents often contain aromatic, or polyaromatic
compounds that tend to be lipophilic. In
the case of colors, they are often potential DNA intercalators
and therefore DNA synthesis disruptors that may lead to induction of neoplasia.
Recognition of these facts in light of the Delaney clause (see below)
has led to evaluation of some of the formerly GRAS dyes and re-categorization
of such compounds as under regulatory control.
As in many other areas of toxicant regulation and legislation the focus
in food legislation is on the prevention of acute effects and on carcinogenesis
potential. In current practice, this
focus on potential carcinogens is largely due to the Delaney clause written
into law in 1958 at the time food safety legislation was being updated to
reflect then current scientific and epidemiological evidence: http://www.cnie.org/nle/crsreports/pesticides/pest‑3.cfm
http://www.acs.ohio‑state.edu/units/cancer/sa97front/Delaney.htm
http://pested.unl.edu/thelabel/tljan98.htm
http://www.rff.org/resources_articles/files/delaney.htm .
The Delaney clause stated that no
compounds shown to be cancer causing in animals or humans would be tolerated at
a measurable level in food or cosmetics. The legislation, however, allowed
those compounds considered GRAS to continue to be used without additional
testing. Note that the "measurable
level" at that time was several orders of magnitude higher than what is
now attainable. In addition, current
toxicological databases include information on a variety of compounds
originally on the GRAS list (such as food colors) that
suggest they may not be suited for chronic, high dose use in
foodstuffs. Note also that the Delaney
clause has been interpreted to apply only to induction of primary carcinogenic
lesions. It does not cover induction of
a state leading secondarily to neoplastic lesions as might be the case for an
endocrine disrupting chemical.
Maturation of the gut plays a major
role in determining just how problematic some of these contaminants may
be. Until maturation allows full
acidification of the stomach and maturation of the blood‑lumen barrier in
the gut, a variety of toxins that are normally acid‑labile and/or
macromolecular can access portions of the digestive tract where they can be
absorbed and taken into general circulation.
This may allow action by acid‑labile enterotoxins, e.g., botulinum toxin, establishment of inappropriate antigenic
tolerance (anergy), and/or alteration of
host/pathogen interactions that may range from deleterious to beneficial.
Diet as a Modulator
Not only do contaminants in food
modify the consumer, constituents in food may modify the consumer's exposure to
and metabolism of certain toxicants. As
we have stated earlier in the course, fiber and lipids may alter gut transit
time, the release of toxicants to the consumer, the enterohepatic
cycling of the toxicant and its metabolites to and from the gut contents, and
the elimination of the intact or metabolized toxicant from the consumer. These constitutents
may modify the kinetics of toxicant entry into the organism or its metabolism
of these toxicants.
Modulation of stomach acidity by
ingestion of a vegetable or high protein diet may also change the dynamics of
toxicant entry into the consumer.
Food content of antioxidants such
as vitamins A, C, and E may directly modulate metabolism in the gut or alter
the redox status of tissues like the liver involved with toxicant
catabolism. This would include any food
components that stimulate phase I or II enzymes; compounds with hormonal
activities that impact hepatic or gut function (steroids, neurotransmitters or
precursors); or compounds with pharmacologic activities that also impact catabolic
tissues (caffeine, salicylic acid, nitrates).
Water
Water purity deals with a host of
potential toxicants: mineral contaminants, organic contaminant chemicals and
biological secondary metabolites, biological breakdown and waste products, and
protein, nucleic acid, and carbohydrate substitutents
deposited in the water supply by natural and manmade causes. While there are natural contaminants in many
water supplies, e.g., ferrous metals, sulfides, plant and aquatic animal waste
products, there are also pollutants arising from anthropomorphic endeavors
(farming, manufacturing, municipal wastes, human wastes). Control of some of these is done using
flocculation or precipitation methods in waste and water treatment plants,
others are minimized by filtration over finely divided solids such as sand or
soil, and some are neutralized or killed by treatment of the water with
broad-spectrum chemical toxins, e.g., ozone, chlorine gas. In all water regulations and purification
processes the idea is to keep as many non-water components as possible out of
the water supply to minimize any toxic loads in the consumers. The popular individual water filters now
being used in homes utilize finely divided charcoal or sintered silica or ion
exchange resins to remove whatever contaminants remain following public water
treatment and the piping of water through ducts that may serve as sources for
pollutants that had been removed prior to introduction into the distribution
system. Such reprocessing and filtration
sytems become all the more important when faced with
the fact that the human population relies for existence on approximately 1% of
the world’s total water resources!

Developmental Stage/Age and
Toxicity
The ability of organisms to cope
with toxic loads is a function of physiological status. That status is a function of gender and age
of development. We have previously covered
some of the issues during fetal development that arise because different
physiological systems mature at differing rates. Moreover, some of these tissues change
functions during development. There is
little wonder that targets for toxic insult shift over developmental time as do
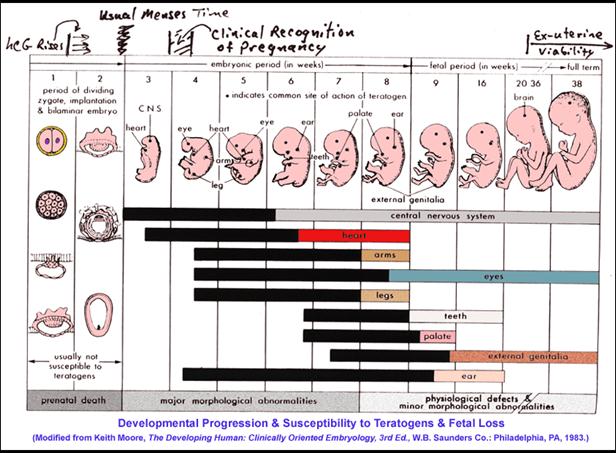
the capacities to counter or repair the damage of such insults. Note the various stages at which various
organs and systems develop and how these coincide with susceptibility to
teratogens.
Physiological Change of Status
in Pregnancy
Now also consider the
physiological status during pregnancy.
Not only has the normal endocrine system shifted into overdrive with
thyroid, adrenal, pancreatic, and hepatic systems producing vastly more
hormones, binding proteins and serum glucose than in the non-pregnant state,
but the fetoplacental unit is also contributing
significant amounts of unique hormones of its own, e.g., hCG, placental lactogen. Moreover,
the maternal body is being altered to handle the energetically demanding processes
of birth and lactation. Major shifts in
energy storage and metabolism are taking place during pregnancy and
lactation. The maternal body’s capacity
to deactivate or metabolize toxicants is also being modified. Hepatic activities change relative to the nonpregnant state.
Kidney blood flow and overall clearance increases. The placenta both binds and metabolizes some
constituents on its own to say nothing of the fetus’ own capacities for toxicant
binding and metabolism. Thus, it is not
surprising to realize that the pregnant female is not equivalent to a somewhat
overweight, non-pregnant female.
Physiological Changes in Old
Age
Similar considerations also
accompany individuals as they age. In
these cases there are usually declines in capacities to accommodate and repair
toxicant insults at the cellular level as well as systemic decreases in
homeostatic system tone. Thus, it
becomes more difficult for the liver to detoxify lipophilic toxicants or for
the kidney to clear enough urine to remove toxicant metabolites. Pancreatic and thyroid functions decline as
does lung function. Epidermal barriers
become thinner and less well hydrated making penetration of toxicants by the
dermal route faster than previously.
Cellular cycle controls and immune surveillance for neoplastic cells
becomes less robust so apoptosis becomes unable to eliminate all possible neoplastically transformed cells. Pathogen
surveillance declines and immune protection against allergens and pathogens
decreases. Interestingly, some of
the best theories on what drives the aging process center on accumulations of
unrepaired toxic insults (especially as initiated and propagated via the
reactive oxygen and reactive nitrogen production and inactivation pathways) as
the primary culprits. Age-related
mobilizations of fat reserves which frees previously non-bioavailable
toxicant molecules also complicates the overall picture of physiological status
in the elderly. It is not at all
surprising that physicians often have a difficult time getting perscription dosages correct in older patients. Nor is it surprising when older individuals
succumb to toxicant loads that are normally well-tolerated by the young or
middle-aged adult population. And while
such considerations may be less easily observed among most other mammals who
often do not survive beyond the onset of old age, the
same processes seem to occur.
Overall, age, developmental, and
reproductive status do make very large contributions to physiological capacity
to absorb, distribute, metabolize, and excrete any toxicants to which an
organism is exposed. They greatly
increase the variability of sensitivities to dose seen within a
population. And they make the processes
of administration or regulation much more challenging than they would be in the
absence of such intrapopulation variability.